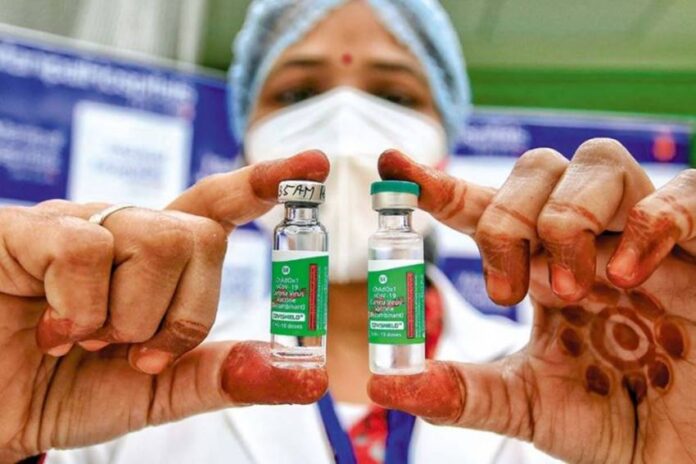
The Drugs Controller General of India (DCGI), country drug regulator, has granted permission for conducting a study on mixing doses of Covaxin and Covishield COVID-19 vaccines.The study and its clinical trials will be conducted by Christian Medical College (CMC) in Tamil Nadu’s Vellore.”Permission has been granted for a research study by CMC Vellore (Tamil Nadu) on mixing of vaccines doses,” NITI Aayog member (health) Dr VK Paul said during a press briefing on Tuesday.Last month, an expert panel of DCGI had recommended conducting a study on mixing doses of Covishield and Covaxin vaccines.The recommendation came after the CMC sought permission to conduct such study.The Subject Expert Committee of the Central Drugs Standard Control Organisation (CDSCO) had recommended granting permission to CMC for conducting the Phase-4 clinical trial covering 300 healthy volunteers for mixing of doses of Covaxin and Covishield.The panel had said that purpose of this study is to assess whether a person can be given two different vaccine shots — one each of Covishield and Covaxin — to complete the vaccination course.
ABOUT US
SACH® - raising the voice of people of Jammu Kashmir since 1940. We are Publishing House of Daily Sach(Urdu Daily). Sach News Network, is one of the oldest News Group of India having its office in Jammu Kashmir, Delhi. Reach us for Latest news on politics, sports, crime, education, real estate, business entertainment and much more. We provide you with the latest breaking news and videos straight from the ground zero.
Contact us: [email protected]
© Sach News Network 2011-2024 | Maintained by Sach Info Tech