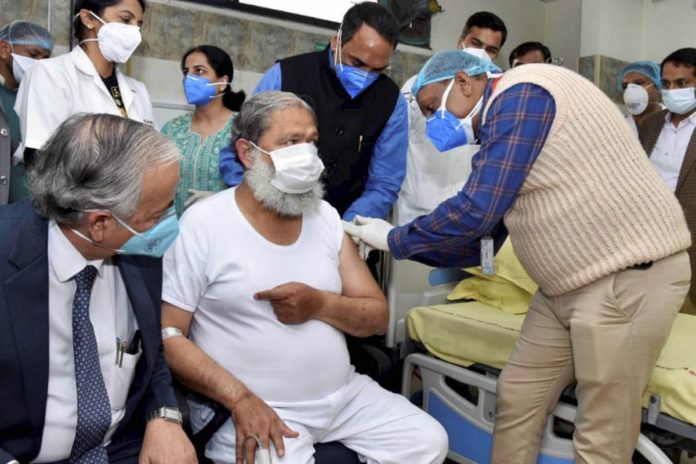
Haryana health minister Anil Vij said on Saturday he has tested positive for the coronavirus disease (Covid-19) and has been admitted to a hospital.
I have been tested Corona positive. I am admitted in Civil Hospital Ambala Cantt. All those who have come in close contact to me are advised to get themselves tested for corona,” he tweeted.
Vij, a Bharatiya Janata Party (BJP) leader, volunteered to be a part of the third phase trial of Covaxin, which started in Haryana on November 20. He was administered a trial dose.However, it’s not immediately clear whether he was administered the vaccine candidate during the trial or a placebo.
Also, the antibodies against the infection build up only after a specific number of days pass after the second dose of the vaccine is taken, since it is a two-dose vaccine, according to a health ministry official who did not want to be named.The official said this was not solely in the context of Vij, who got just one dose — of either the vaccine or a placebo.Vij’s media coordinator, Vijender, Chauhan said the minister was in Chandigarh on Friday afternoon and had reported fatigue later. “He got tested himself today morning and the result is positive. The staff will be tested today itself,” Chauhan said.
Bharat Biotech had said that phase 3 trials would involve a total of 26,000 people and be conducted at 25 centres across the country. “After successful completion of the interim analysis from the phase 1 and 2 clinical trials of Covaxin, Bharat Biotech received Drug Controller General of India (DCGI) approval for phase 3 clinical trials in 26,000 participants in over 25 centres across India,” the company had said in a statement. This will be India’s first phase 3 efficacy study for a Covid-19 vaccine, and the largest such trial ever conducted in the country, the statement added.According to top officials of the National Institute of Cholera and Enteric Diseases (ICMR-NICED) in Kolkata, the results of the phase-III trial of the indigenously developed coronavirus vaccine candidate, Covaxin, will be available in November 2021. The interim report may be available by May 2021 at the earliest, they added