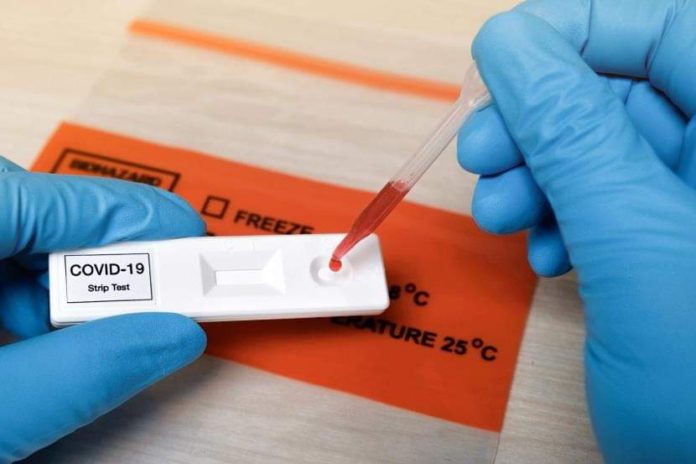
SCientists have developed a new simplified COVID-19 diagnostic test that can be ramped up to analyse thousands of blood samples at labs lacking the resources of commercial medical centres, an advance that may help scale up testing for the deadly disease. According to the researchers from the University of North Carolina in the US, there is a need for widely applicable surveillance testing to gain a better understanding of infection rates, especially the number of people with mild or no symptoms of COVID-19, who can still be carriers of the disease. The study, published in the journal Science Immunology, describes a new blood test which pinpoints the immune system molecules called antibodies in infected individuals that specifically target a unique piece of the novel coronavirus SARS-CoV-2’s spike protein. This unique protein on the virus called the receptor binding domain, or RBD, enables the virus to gain entry into host cells, the study noted. According to the researchers, their RBD-based antibody test can measure the levels of this protein, which they said correlates to the levels of the body”s neutralising antibodies that provide immunity in infected individuals. Since the RBD of the spike protein in SARS-CoV-2 is not shared among other known human or animal coronaviruses, the scientists said antibodies against this domain are likely to be highly specific to the virus, and can reveal if an individual has been exposed to it. When the researchers tested blood collected from people exposed to other coronaviruses, none had antibodies to the RBD of SARS-CoV-2, the study noted. “Our assay is extremely specific for antibodies to the virus that causes COVID-19, which is not the case for some currently available antibody tests,” said study co-author Aravinda de Silva from the University of North Carolina. Based on the findings, the researchers support the use of their RBD-based antibody assay for population-level surveillance, and as an estimate of the neutralising antibody levels in people who have recovered from SARS-CoV-2 infection. “We are now further streamlining our test into an inexpensive assay, so that instead of the test taking four to five hours to complete, our assay could be completed in about 70 minutes without compromising quality,” said study co-author Prem Lakshmanane from the University of North Carolina. In the study, the scientists designed new antigens, which are protein parts of the virus that generate an immune response in people, and used a large panel of SARS-CoV-2 patients and control human and animal samples. From day nine after the onset of symptoms and thereafter, the scientists said the new test allowed them to accurately identify RBD-based antibodies to SARS-CoV-2. The scientists then compared the RBD-based antibody levels in patients with the levels of neutralising antibodies detected in another sensitive assay. “We observed a robust correlation between levels of RBD-binding antibodies and SARS-CoV-2 neutralising antibodies in individual samples,” Lakshmanane said. This means the RBD-assay not only identifies people exposed to SARS-CoV-2, but it can also be used to predict levels of neutralising antibodies and to identify potential donors for plasma therapy, he explained. “It”s important for researchers to stay engaged, to monitor antibody responses and other biological details, and to fine tune assays to meet the different needs of individual patients, the public health community, and vaccine developers,” Lakshmanane said.
ABOUT US
Sach News® - raising the voice of people of Jammu Kashmir since 1940. We are Publishing House of Daily Sach (Urdu Daily). Sach News, is one of the Oldest News Group of India having its office in Jammu Kashmir, Delhi. Reach us for Latest news on politics, sports, crime, education, real estate, business entertainment and much more. We provide you with the latest breaking news and videos straight from the ground zero.
Contact us: [email protected]
© Sach News Network 2011-2024 | Maintained by Sach Info Tech